Molecular analysis of Plasmodium invasion of red blood cells: ligand-receptor interactions and signal transduction
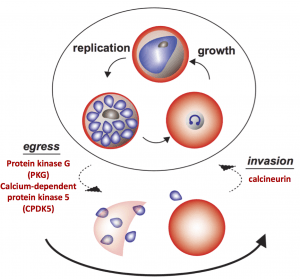
A major focus of our laboratory has been the elucidation of the mechanism by which Plasmodium parasites invade host red blood cells (RBCs), within which they proliferate or become transmissible sexual forms. Invasion occurs via a complex mechanism, with many features unique to apicomplexan parasites, beginning with attachment to the host RBCs, followed by the formation of a tight junction, active entry into the RBC and resolution of the parasitophorous vacuole (PV). Entry involves multiple and specific ligand-receptor interactions, which are known as invasion pathways. The merozoite surface is directly exposed to host immunity making invasion ligands potential candidates for vaccine development.
We are working to identify specific ligand-receptor interactions in different Plasmodium spp. to inform vaccine development and to understand the biological implications of the use of alternative ligand-receptor pairs, known as invasion pathways.
Papers of Interest:
The Molecular Basis of Erythrocyte Invasion by Malaria Parasites. (Review)
Cowman AF, Tonkin CJ, Tham WH, Duraisingh MT. Cell Host Microbe. 2017 Aug 9;22(2):232-245.
Parasite Calcineurin Regulates Host Cell Recognition and Attachment by Apicomplexans.
Paul AS, Saha S, Engelberg K, Jiang RH, Coleman BI, Kosber AL, Chen CT, Ganter M, Espy N, Gilberger TW, Gubbels MJ, Duraisingh MT. Cell Host Microbe. 2015 Jul 8;18(1):49-60.
Red blood cell determinants of malaria infection: invasion, trafficking, growth and sexual development
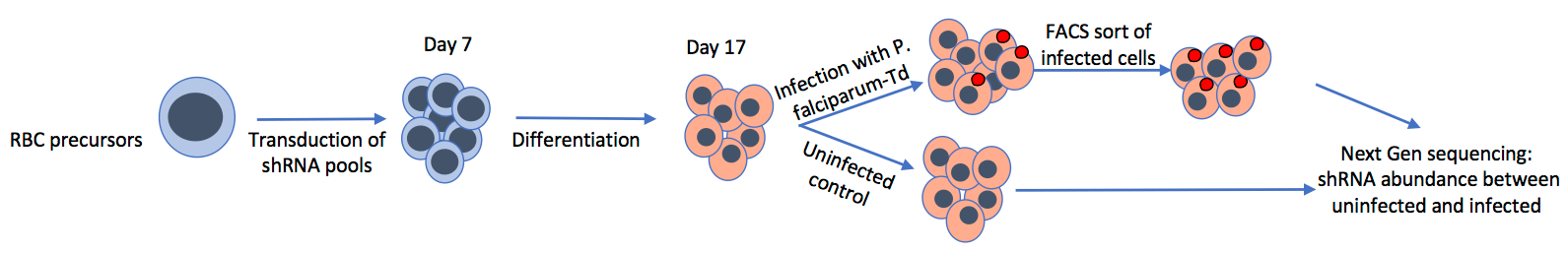
Plasmodium parasites have exerted a huge selective force on the human host over a long period of evolution, leaving strong signatures of selection in the human genome, including numerous red blood cell disorders (e.g. sickle cell disease). Most malaria research efforts have targeted parasite proteins, but a direct focus on identifying host determinants of infection represents a novel approach for elucidating critical host-pathogen interactions.
We have developed in vitro genetic approaches for the identification of critical red blood cell genes involved in malaria infection. The differentiation of primary hematopoietic stem cells and immortalized cell-lines into reticulocytes and young erythrocytes makes the enucleated red blood cell amenable to genetic analysis, through targeted lentivirus-based methods for gene knockdown.
We have undertaken forward genetic screens to identify red blood cell determinants of various aspects of malaria infection, including invasion, growth, protein trafficking and sexual development.
Papers of interest:
Host-parasite interactions that guide red blood cell invasion by malaria parasites. (Review)
Paul AS, Egan ES, Duraisingh MT. Curr Opin Hematol. 2015 May;22(3):220-6.
Egan ES, Jiang RH, Moechtar MA, Barteneva NS, Weekes MP, Nobre LV, Gygi SP, Paulo JA, Frantzreb C, Tani Y, Takahashi J, Watanabe S, Goldberg J, Paul AS, Brugnara C, Root DE, Wiegand RC, Doench JG, Duraisingh MT. Science. 2015 May 8;348(6235):711-4.
In vitro genetic analysis of an erythrocyte determinant of malaria infection.
Bei AK, Brugnara C, Duraisingh MT. J Infect Dis. 2010 Dec 1;202(11):1722-7.
Epigenetic regulation of virulence gene expression and sexual development in malaria parasites
The laboratory has a long-standing interest in the regulation of variant expression of virulence gene families in P. falciparum, to determine how a parasite persists in human infections and evades the immune system and switches from asexual proliferation to sexual development for transmission. The mechanistic basis for this critical process has been determined to involve epigenetic regulation of transcriptional pathways, involving the interplay of several epigenetic regulators.
Our current studies aim to establish a comprehensive functional understanding of the role of P. falciparum proteins involved in epigenetic regulation, with a particular focus on histone deacetylases, to reveal the biological processes that they regulate within the laboratory. These studies involve a combination of parasite genetics, transcriptomics, proteomics and single-cell approaches.
Papers of interest:
Epigenetic Regulation of Virulence Gene Expression in Parasitic Protozoa. (Review)
Duraisingh MT, Horn D. Cell Host Microbe. 2016 May 11;19(5):629-40.
A Plasmodium falciparum histone deacetylase regulates antigenic variation and gametocyte conversion.
Coleman BI, Skillman KM, Jiang RHY, Childs LM, Altenhofen LM, Ganter M, Leung Y, Goldowitz I, Kafsack BFC, Marti M, Llinás M, Buckee CO, Duraisingh MT. Cell Host Microbe. 2014 Aug 13;16(2):177-186. doi: 10.1016/j.chom.2014.06.014.
Biology and determinants of in vitro proliferation of Plasmodium vivax
With the call for the eradication of malaria, interest in understanding the biology of all human malaria parasites, including P. vivax, is increasing. Due to the ability of P. vivax to hide in the liver, they may persist even when P. falciparum is eliminated, and are therefore of great public health importance. This parasite has been understudied, in part due to the lack of in vitro culture system, leaving extensive gaps in knowledge of its strategies and mechanisms of infection, that are distinct to that of P. falciparum.
P. vivax is limited to the invasion of reticulocytes. We are focused on identifying the ligand-receptor interactions that are required for parasite invasion and result in reticulocyte tropism, and to characterize those with potential for vaccine development.
We are developing and employing assays to study invasion, antibody inhibition and drug susceptibility, both using cryopreserved isolates in Boston and in field studies in India.
Much effort is going into establishing the determinants of P. vivax growth and red blood cell tropism, with the long-term goal of establishing in vitro P. vivax culture that will facilitate biological and genetic studies.
Papers of interest:
Rangel GW, Clark MA, Kanjee U, Lim C, Shaw-Saliba K, Menezes MJ, Mascarenhas A, Chery L, Gomes E, Rathod PK, Ferreira MU, Duraisingh MT. Antimicrob Agents Chemother. 2018 Jan 29. pii: AAC.02519-17. doi: 10.1128/AAC.02519-17.
Insights into an Optimization of Plasmodium vivax Sal-1 In Vitro Culture: The Aotus Primate Model.
Shaw-Saliba K, Thomson-Luque R, Obaldía N 3rd, Nuñez M, Dutary S, Lim C, Barnes S, Kocken CH, Duraisingh MT, Adams JH, Pasini EM. PLoS Negl Trop Dis. 2016 Jul 27;10(7):e0004870
Zoonotic infections of Plasmodium spp. and Babesia spp.
We are interested in identifying the molecular basis of the tropism of Plasmodium spp. parasites for different primate species, to explore the potential for cross-species transmission to humans, and define the molecular changes that facilitated the emergence of the extant human malaria parasites.
We have established the zoonotic primate malaria parasite, P. knowlesi, as an in vitro model to study red blood cell invasion, that possesses powerful genetics and cell biology, that we are now able to exploit for detailed imaging of parasite invasion and forward genetic approaches.
We have initiated studies of the biology of Babesia spp., that are evolutionarily closely related to Plasmodium spp.. Traditionally an important veterinary disease, babesiosis has recently been recognized as an emerging source of zoonotic infection, with infections resulting from tick transmission. Several Babesia spp. can be robustly cultured in vitro and we have established genetic methods for their functional analysis using genetics and chemical genetics.
Papers of interest:
Lim C, Dankwa S, Paul AS, Duraisingh MT. Cold Spring Harb Perspect Med. 2017 Nov 1;7(11).
Extensive Shared Chemosensitivity between Malaria and Babesiosis Blood-Stage Parasites.
Paul AS, Moreira CK, Elsworth B, Allred DR, Duraisingh MT.
Antimicrob Agents Chemother. 2016 Jul 22;60(8):5059-63. doi: 10.1128/AAC.00928-16.
Ancient human sialic acid variant restricts an emerging zoonotic malaria parasite.
Dankwa S, Lim C, Bei AK, Jiang RH, Abshire JR, Patel SD, Goldberg JM, Moreno Y, Kono M, Niles JC, Duraisingh MT. Nat Commun. 2016 Apr 4;7:11187.
Expansion of host cellular niche can drive adaptation of a zoonotic malaria parasite to humans.
Lim C, Hansen E, DeSimone TM, Moreno Y, Junker K, Bei A, Brugnara C, Buckee CO, Duraisingh MT. Nat Commun. 2013;4:1638. doi: 10.1038/ncomms2612.
Field studies with parasite isolates with collaborators in Goa, India and Dakar, Senegal
[googlemaps https://www.google.com/maps/d/u/0/embed?mid=1mskwBsxc2YIklKgFRHbBd5wJCfaJVQg_&w=640&h=480]
An important facet of our research is the ability to understand the significance of our laboratory findings with clinical isolates through collaborations with researchers/friends in malaria endemic regions.
Papers of Interest:
Malaria in India: The Need for New Targets for Diagnosis and Detection of Plasmodium vivax.
Patankar S, Sharma S, Rathod PK, Duraisingh MT. Proteomics Clin Appl. 2017 Nov 29.
Bei AK, Ahouidi AD, Dvorin JD, Miura K, Diouf A, Ndiaye D, Premji Z, Diakite M, Mboup S, Long CA, Duraisingh MT.
J Infect Dis. 2017 Jul 15;216(2):267-275.
WAMIN Consortium Authors, Ahouidi AD, Amambua-Ngwa A, Awandare GA, Bei AK, Conway DJ, Diakite M, Duraisingh MT, Rayner JC, Zenonos ZA. Trends Parasitol. 2016 Apr;32(4):274-83.
Shaw-Saliba K, Clarke D, Santos JM, Menezes MJ, Lim C, Mascarenhas A, Chery L, Gomes E, March S, Bhatia SN, Rathod PK, Ferreira MU, Catteruccia F, Duraisingh MT. Int J Parasitol. 2016 Oct;46(11):679-83.
Reticulocyte Preference and Stage Development of Plasmodium vivax Isolates.
Lim C, Pereira L, Saliba KS, Mascarenhas A, Maki JN, Chery L, Gomes E, Rathod PK, Duraisingh MT. J Infect Dis. 2016 Oct 1;214(7):1081-4.
